HELIUM (He): GASEOUS ALL-ROUNDER
Liquid helium is used wherever extremely low temperatures (below minus 200 degrees Celsius) must be attained but conventional refrigeration systems are not cost effective. Helium cooling is used with magnetic resonance imaging (MRI) and nuclear magnetic resonance (NMR) spectroscopy systems. In welding and cutting technology, gaseous helium plays an important role in many processes. Helium is also in demand in the electronics industry and for glass manufacturing. Helium-filled balloons rise into the sky for research purposes, among other things. It is also used in airbag generators and in the search for leaks. And divers need mixtures with a share of helium in order to dive to great depths.
Helium has the second lowest molecular weight of all gases after hydrogen. In addition, it is an absolutely inert gas – so even at high temperatures, it does not form chemical compounds. Liquid helium has the lowest boiling point of all gases (minus 269 Celsius), which makes it is the coldest possible liquid. Its small atomic diameter enables helium to penetrate the smallest of openings and pores.
Helium was discovered by the Frenchman Jules Janssen during a total solar eclipse in 1868: he observed light yellow lines in the spectrum of the Sun, which indicated an as yet unknown element. That same year, English researcher Norman Lockyer confirmed Janssen’s observation. Together with his colleague Edward Frankland, he proposed to call the new element helium, which roughly means “sun metal.” In 1882, the Italian Luigi Palmieri was able to prove the existence of helium on the Earth for the first time by submitting an extrusive volcanic rock to spectral analysis.
Early in the 20th century, large quantities of helium were discovered in natural gas fields in the United States. Thanks to their natural gas reserves, Canada, Poland and Russia, and eventually Algeria and Qatar, also all became international helium suppliers. In 2016, a huge supply of helium was discovered in Tanzania. Nevertheless, the ever increasing consumption of helium has already led experts to speak of a helium shortage on multiple occasions. Then as now, countermeasures include plants for recovering helium and more sustainable practices for handling this noble gas.
Occurrence:
Mass fraction in the Earth’s atmosphere is 5.25 ppm, second most common element in the universe, most of the Earth’s supply in natural gas, but also in crude oil, volcanic rock and the Earth’s atmosphere
Chemical properties:
Colorless, odorless, non-toxic noble gas with a neutral taste, the only substance that does not solidify under standard atmospheric pressure even at absolute zero (- 273.15 °C)

THIN-FILM SOLAR CELLS
Adaptable energy production

GAS AS SIMPLE, FAST AND SAFE FILLING MATERIAL FOR BALLOONS
Buoyant mood

HELIUM CARRIES MEASUREMENT TECHNOLOGY INTO THE ATMOSPHERE
Predicting the weather

GASES IN RESEARCH
Nitrogen and helium for cutting-edge research
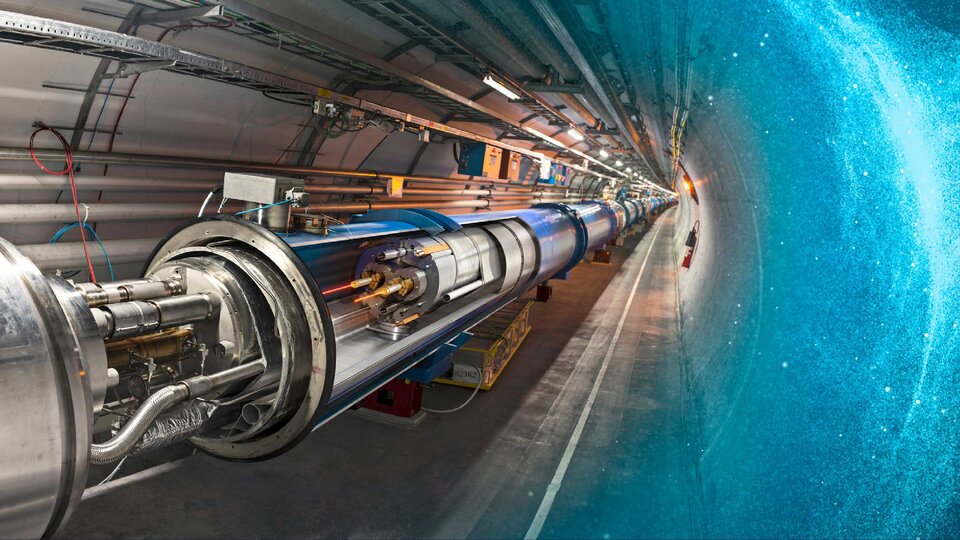
PARTICLE ACCELERATOR
Cooling for magnets

GASES FOR FIRE EXTINGUISHERS
Weld, test, fill

SOLVING CRIMINAL CASES
Gases for crime-fighting

WEATHER RESEARCH
In the eye of the storm
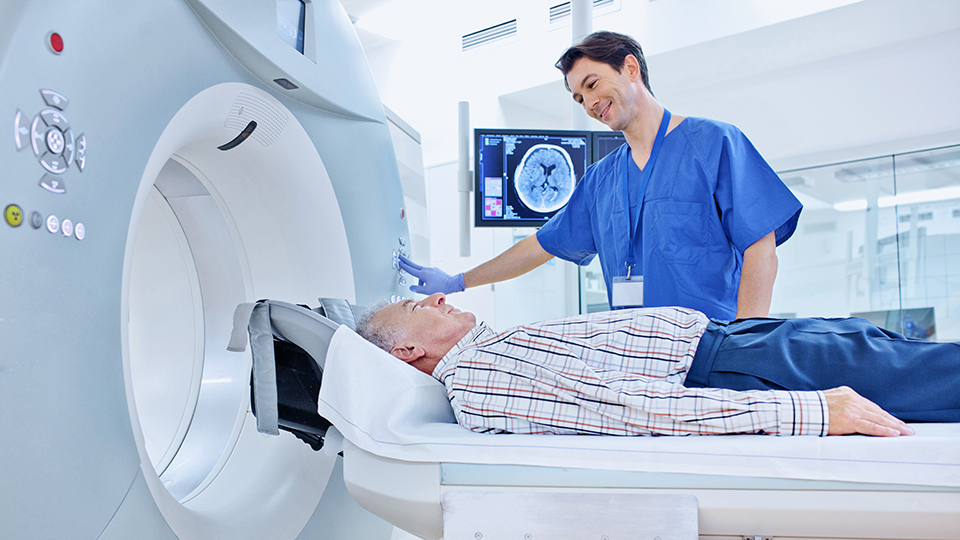
MAGNETIC RESONANCE IMAGING (MRI)
Detailed insight

SPACE RESEARCH
Gases for Rosina

DIVING
Safety at great depths

AIRBAGS NEED MORE THAN JUST AIR
Cushy lifesavers

